90%
of stored biosamples sit idle due to poor metadata, consent limits, and silos.
$113–136k
per patient trial cost, paid again every time idle samples force new recruitment.
66%
patient representatives support broad consent enabling future sample use.
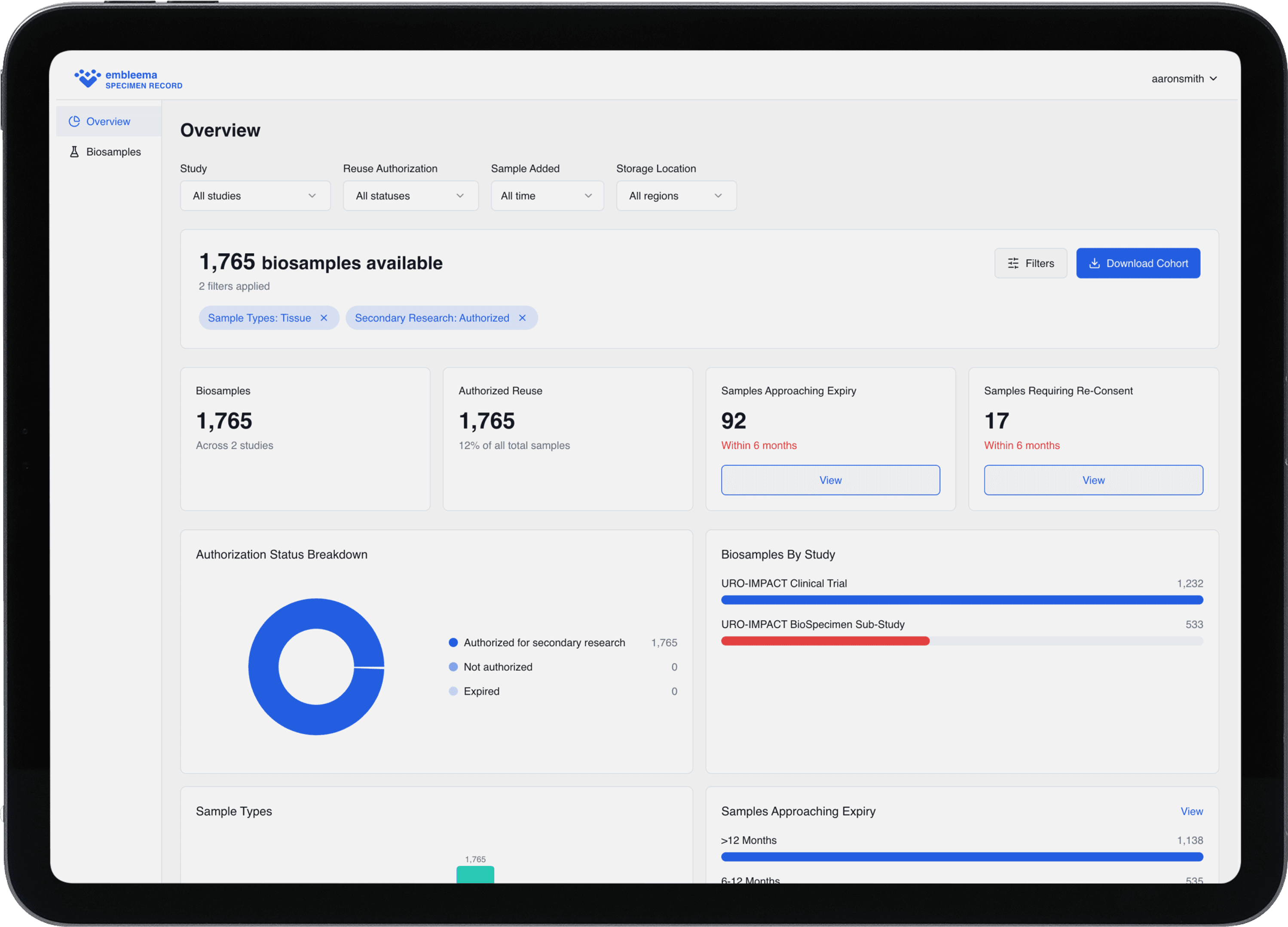
Real-time visibility into storage conditions
Automated re-consent workflows
Global regulatory compliance
Integrated Specimen Management Suite powered by Embleema & Astoriom
Clinical data & consent intelligence
Embleema centralizes clinical, patient, and sample data collection built on a real-time consent management platform, creating a completely clinically characterized digital profile for every sample.
Centralized consent
Audit trail
Authorized use
Metadata enrichment
Re-contact workflows
Global biospecimen stewardship
Astoriom delivers high-quality specimen continuity across the entire development and storage lifecycle, from ambient to cryogenic biorepository services through full stability storage.
Testing
Storage & stability
Lifecycle continuity
Retrieval
Integrated visbility
Discover a unified view of every sample — its metadata, clinical context, consent status, and real time visibility.
Operational efficiency
Reduce recollection, minimize manual reconciliation, and accelerate access for approved research.
Compliance & governance
Built-in guardrails automate enforcement of authorized use and trigger re-consent workflows.
Scientific insight
Discover new opportunities for underutilized samples through lifecycle metadata and integrity control.
Powering Global Clinical Research
Astoriom enables compliant, end-to-end biospecimen management for global research programs.