
Article
From Storage to Reuse: The New Phase of Biosample Management
Reflections from SCOPE Europe 2025
At this year’s SCOPE Europe, one number kept coming up in hallway conversations and session Q&As: only about 6% of biosamples are ever reused.
For an industry built on discovery, that means 94% of potential insight sits frozen — literally — in storage. The question isn’t whether we can collect more, but whether we can finally make what we already have reusable, responsibly.
And that responsibility starts with consent — knowing exactly what patients have agreed to, and being able to trace, manage, and honor those permissions as studies evolve. Across the conference, it was clear: consent is no longer just a compliance document. It’s becoming the foundation for sustainable reuse and trust between patients, sites, and sponsors.
A clearer path is emerging
In conversations with sponsors, sample managers, and data governance teams, a three-phase maturity curve is starting to take shape. It’s a framework that helps organizations locate where they are today — and where they’re heading next.
Phase 1: Centralizing consent
Most organizations are here today. They’re working to pull together every version of every consent form — across studies, countries, and document types — just to answer a simple question: What can we actually do with these samples?
That means reconciling signed PDFs from old clinical studies, scanned paper forms from investigator sites, and digital eConsent records collected under different templates and languages. Each version can carry slightly different wording about secondary use, storage duration, or genomic analysis — details that determine whether a sample can legally and ethically be reused.
It’s painstaking work, but it’s where transformation begins. Without a unified repository of consent terms linked to each biosample ID, teams are operating on assumptions, not certainty. Phase 1 is about visibility — consolidating fragmented consent data so reuse decisions are informed, compliant, and defensible.
Phase 2: Connecting the lifecycle
A smaller group of teams are now moving into Phase 2 — building an end-to-end view of the biosample lifecycle.
They’re linking consent data with operational metadata — connecting what patients agreed to with when and how each sample was collected, processed, shipped, and stored.
That often means pulling data from LIMS, biobank inventory systems, and EDC platforms, and reconciling details like collection dates, preparation protocols, storage temperatures, and chain-of-custody records with the corresponding consent terms.
When these pieces come together, every sample carries both its scientific context and its ethical provenance. Compliance teams can instantly verify that reuse aligns with consent, and researchers can identify samples suitable for new analyses without triggering lengthy re-checks.
This kind of traceability not only de-risks reuse — it raises the value of each biosample, turning what was once static inventory into a searchable, reusable research asset.
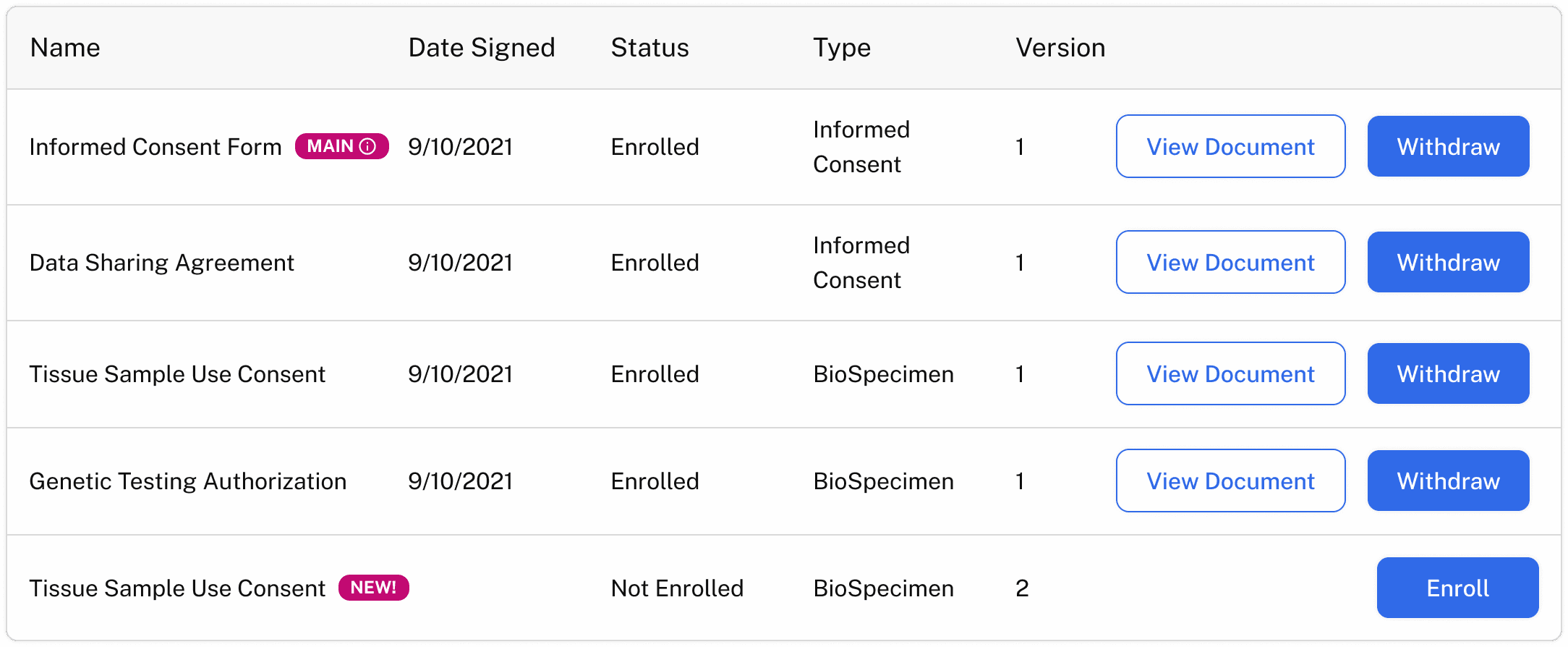
Phase 3: Enabling dynamic consent
The most forward-thinking organizations are entering Phase 3 — where consent becomes living and digital.
Patients can view and manage their consent through a secure portal, with any updates — from granting permission to withdrawing — syncing automatically across sponsor, site, and biobank systems.
Behind the scenes, integrations with eConsent platforms and data-management tools keep every record and access permission current.
For sponsors, that means instant clarity on which samples are reusable or restricted.
For patients, it means transparency and control over how their samples continue to support research.
This is the foundation for true ethical reuse — where patients retain agency and sponsors gain the trust and clarity to accelerate discovery. Dynamic consent turns reuse from a compliance task into a sustainable, trust-based practice that’s fast becoming the industry’s North Star.
Looking ahead
What stood out most at SCOPE wasn’t just technology — it was intention.
Sponsors and sites want to do right by patients and by science. The conversation is shifting from “how do we store more?” to “how do we reuse responsibly?”
The biggest untapped opportunity in life sciences isn’t new data — it’s reusing the data and samples we already have, responsibly.
As the field advances, the organizations that turn consent into a living, verifiable process will be the ones to unlock faster studies, reduce waste, accelerate research, and ultimately benefit from stronger patient partnerships.
As the conversations at SCOPE Europe showed, the industry is ready to move from storage to reuse.
The question is no longer if it will happen — but how soon.
About Embleema
At Embleema, we believe consent is more than compliance — it’s the currency of trust that allows data and samples to move safely through time.
Our technology platform connects biosample data with dynamic, patient-linked consent, helping sponsors, sites, and patients manage consent seamlessly across the research lifecycle — from biobanks to real-world evidence — enabling ethical reuse, regulatory compliance, and accelerated discovery.