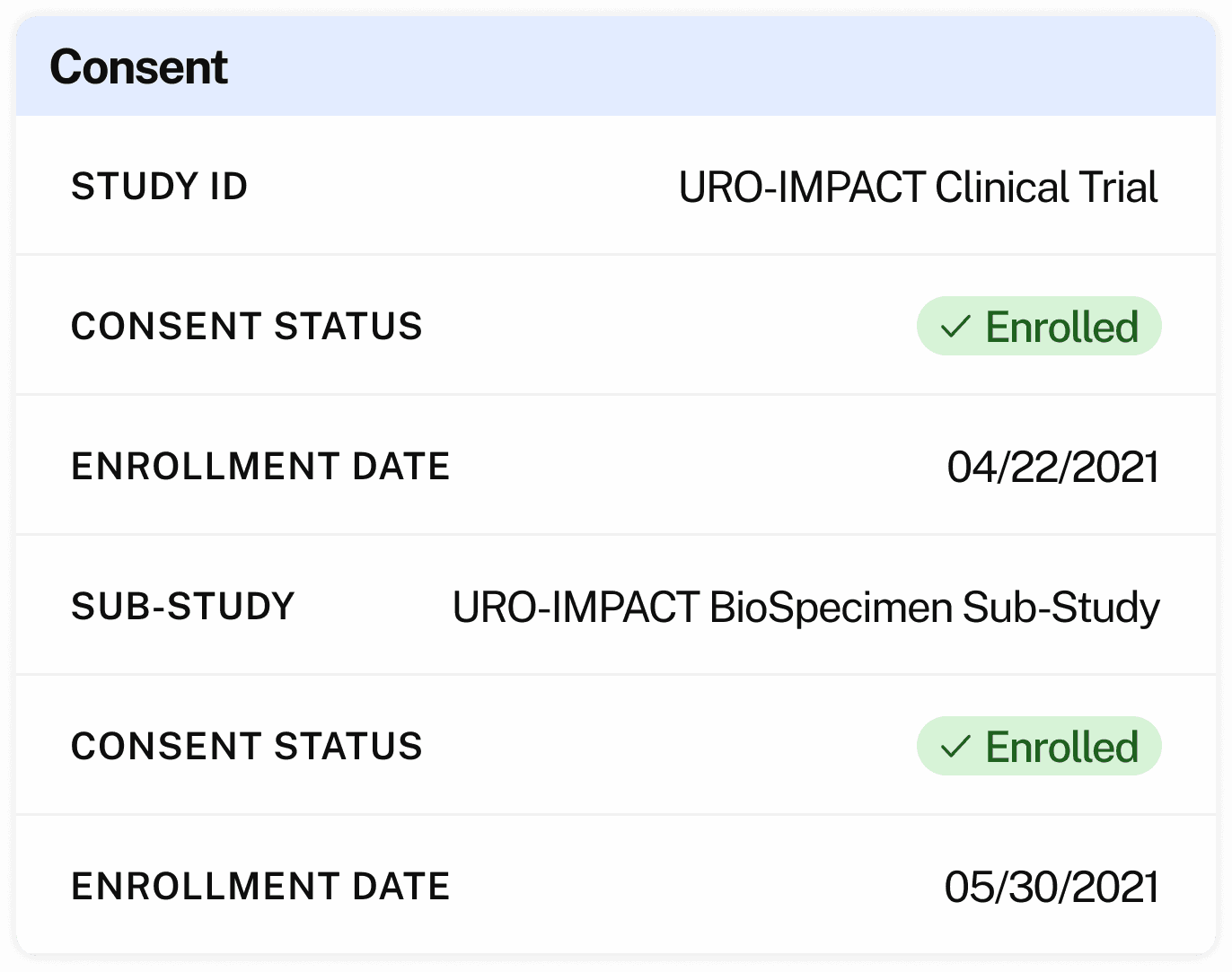
Centralize consent across versions, trials, countries
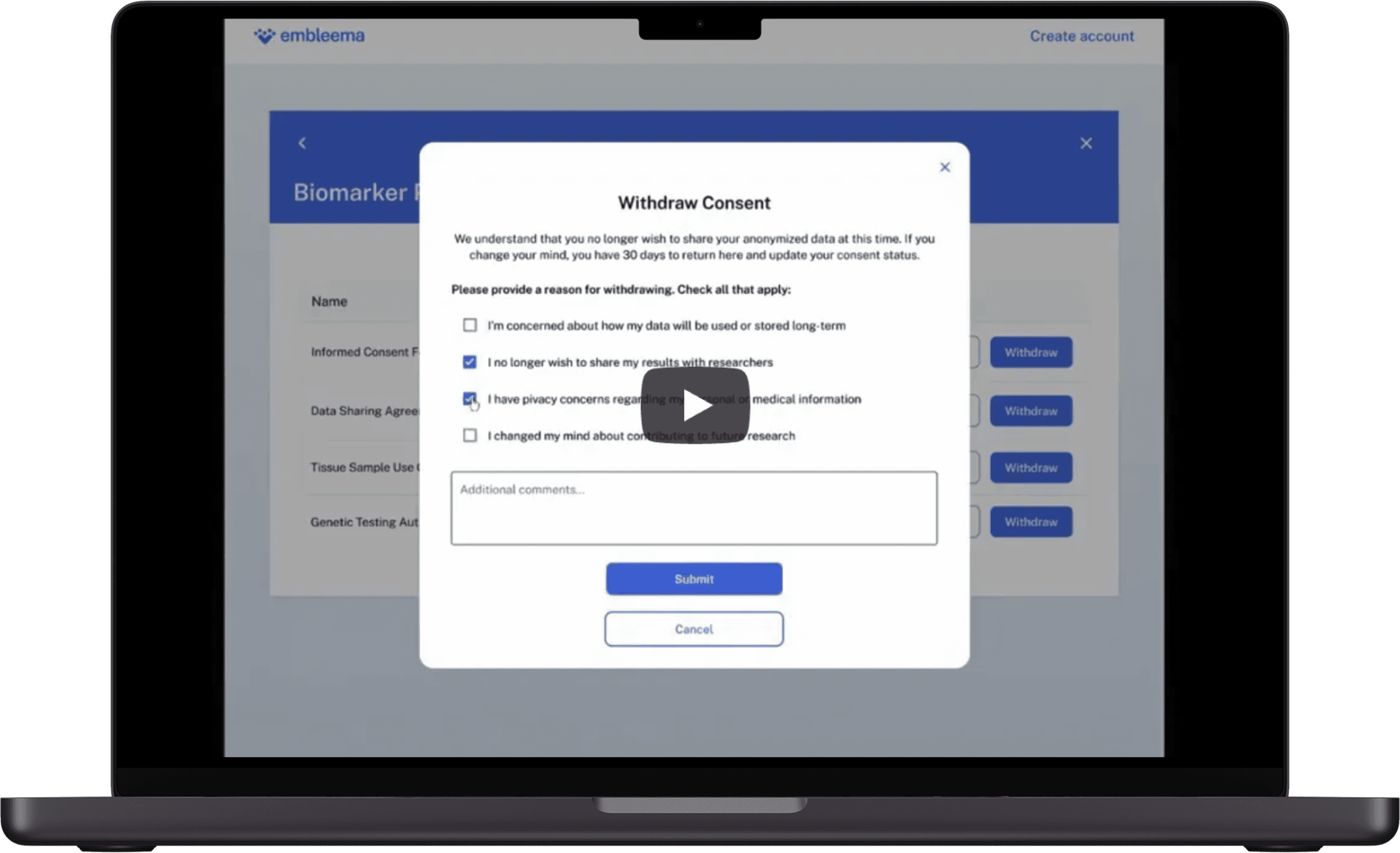
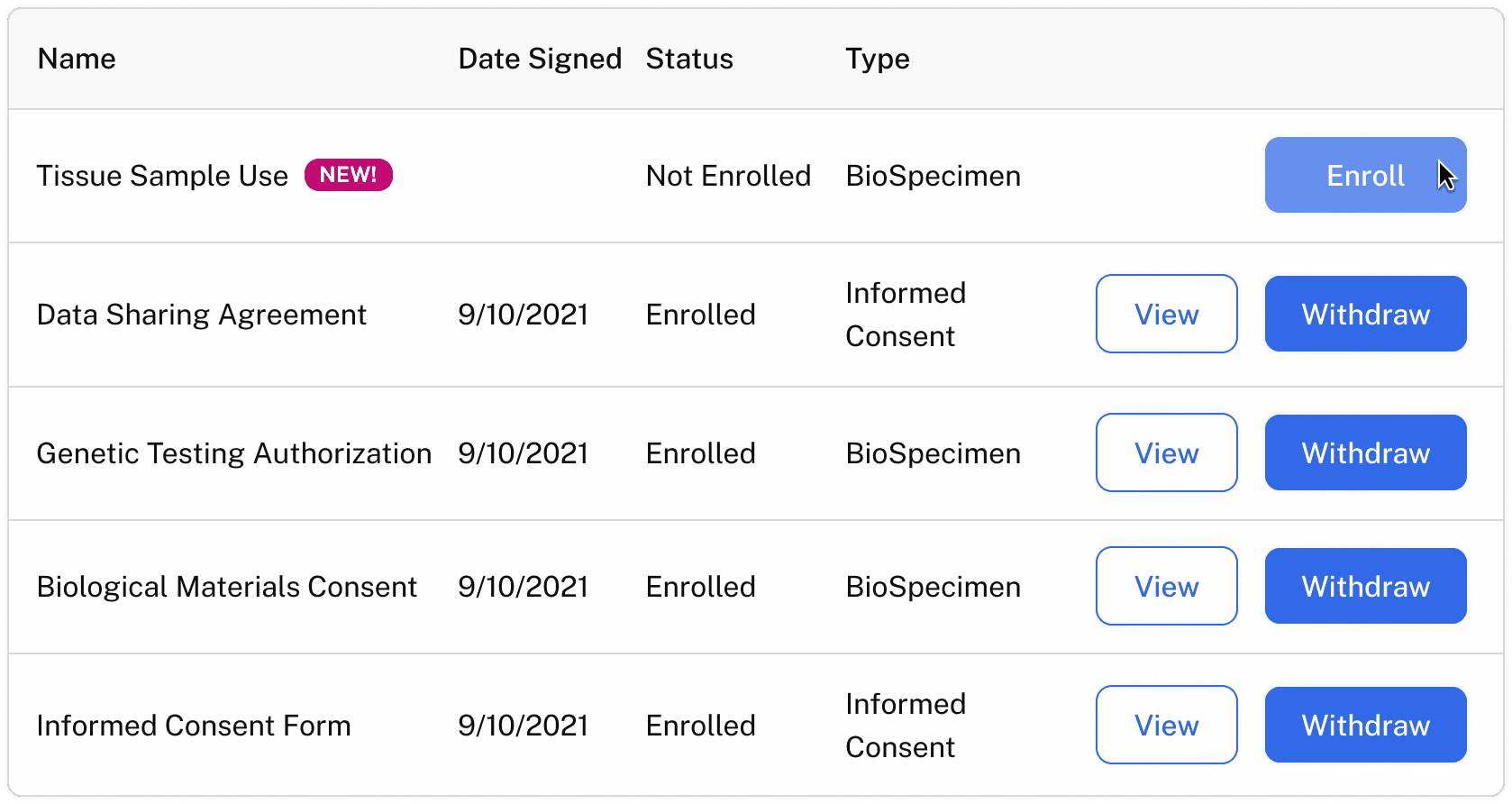
Participant self-service to update, withdraw, or re-consent for reuse
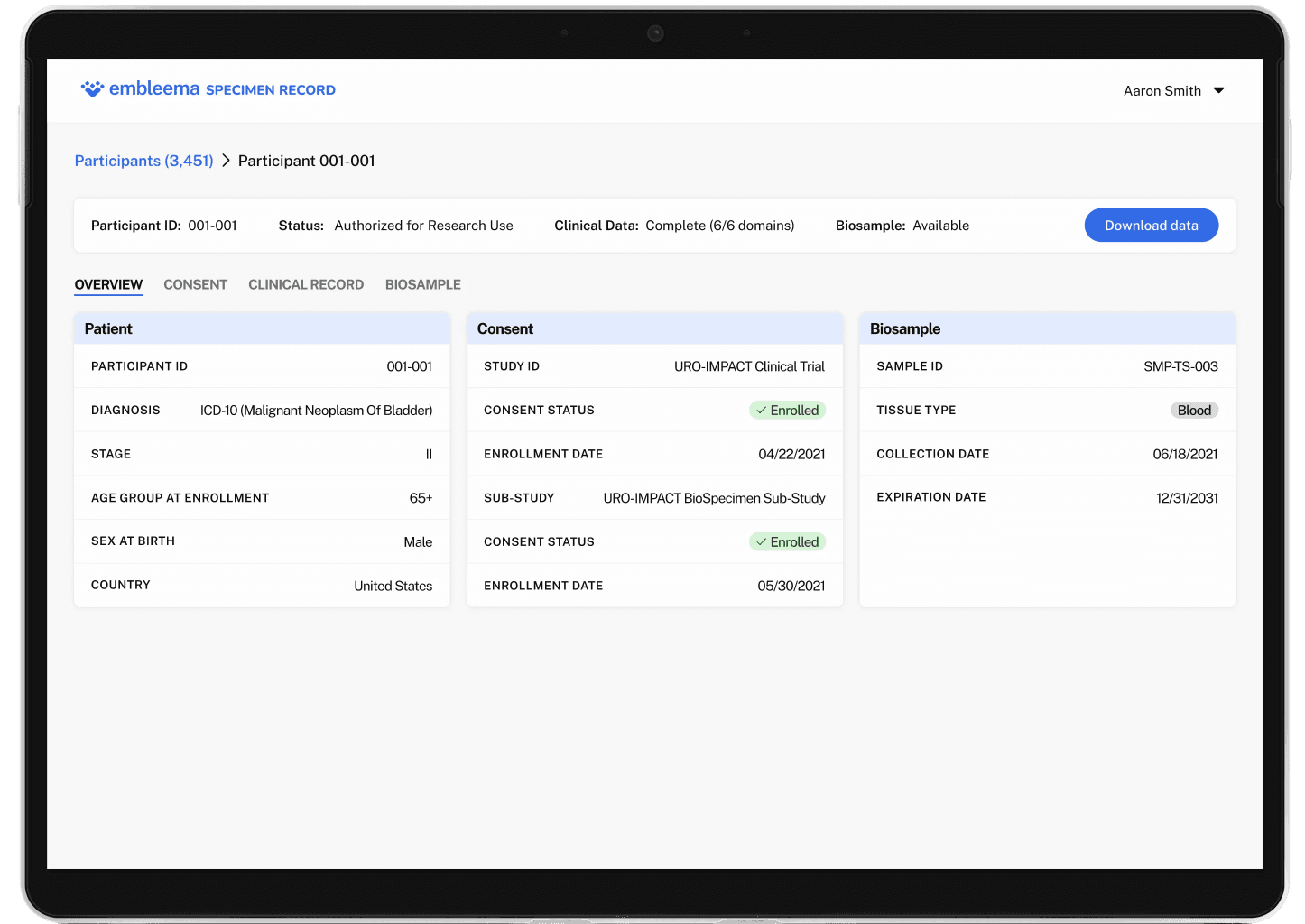
Consent status linked to biospecimen and clinical metadata
Secure identity verification even after site closure
One record that unifies consent, participant, clinical, and sample data
Introducing the Integrated Specimen Management Suite
We've partnered with Astoriom to bridge the gap between biological sample management and digital consent. Ensure every sample in your biobank is matched with active, verified patient consent in real-time.
Thought leadership


Turn patient consent into a strength, not a risk
Get a walkthrough of dynamic consent for active and closed studies.